Educator Onboarding
LEO Art Challenge Workshop
ICE 2019: Satellite Tracking, Orbits, and Modeling
SEEC 2019: Satellite Tracking, Orbits, and Modeling
Workshop:ITEC Trek-a-Sat
Workshop: 2018-01-27 Yerkes
Workshop: 2017-10-28 Carthage-Yerkes Electrostatics in Space
Workshop: 2017-06-29-BTCI-Life in Space!
Workshop: 2017-03-11 Yerkes
Workshop: 2017-02-07 SEEC
Workshop: 2017-01-28 Yerkes
Tools You Might Use
Educational Learning
Standards
Documentation
Chemical Bonds
Programação
-
Written by: Suzanne Monir, EIS Education Team Member, December 2015
Title of Lesson: Chemical Bonds
Topic: Intermolecular and Intramolecular bonds, Surface tension, Capillary action
Grade (Age) Level: High School (Ages 14-18), UniversityThis course module is a summary of various intramolecular and intermolecular bonds that exist in chemistry with applications to surface tension and capillary action. The impact of bonding should be considered in its own right or as an integral aspect of the majority of microgravity experiments.
Course Index:
-
All biological compounds are impacted directly or indirectly by hydrogen bonds.
Proteins, which make up so much of our structures and enzymes, are full of hydrogen bonds.

Similarly, carbohydrates and nucleic acids also are dependent on hydrogen bonds in their 3-Dimensional structure.
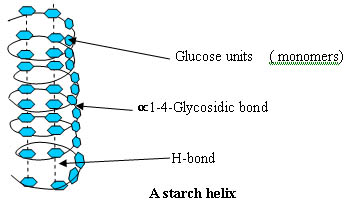
And Deoxyribonucleic acid (DNA):

Hydrogen bonds give us many of the coolest things in nature.
- Why water bugs can walk on water
- The reason geckos can walk on walls
- The reason water in plant vesicles seem to go AGAINST gravity in capillary action
- Why paper towels "soak" water
Surface Tension: (from Chemwiki)
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface tension properties.
Introduction
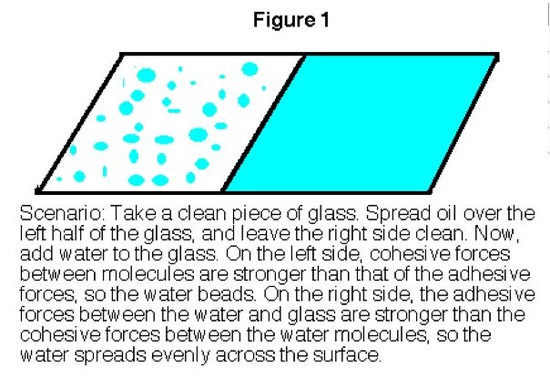
Whether you know it or not, you already have seen surface tension at work. Whenever you fill a glass of water too far, you may notice afterward that the level of the water in the glass is actually higher than the height of the glass (Figure 1 below). You may have also noticed that the water that you spilled has formed into pools that rise up off the counter. Both of these phenomena are due to surface tension.
How Does It Work?
In a sample of water, there are two types of molecules. Those that are on the outside, exterior, and those that are on the inside, interior. The interior molecules are attracted to all the molecules around them, while the exterior molecules are attracted to only the other surface molecules and to those below the surface. This makes it so that the energy state of the molecules on the interior is much lower than that of the molecules on the exterior. Because of this, the molecules try to maintain a minimum surface area, thus allowing more molecules to have a lower energy state. This is what creates what is referred to as surface tension. An illustration of this can be seen in figure 1 below.
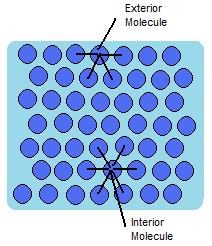
The water molecules attract one another due to the water's polar property. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to "stick" together. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Same goes for other liquids, even hydrophobic liquids such as oil. There are forces between the liquid such as Van der Waals forces that are responsible for the intermolecular forces found within the liquid. It will then take a certain amount of energy to break these forces, and the surface tension. Water is one liquid known to have a very high surface tension value and is difficult to overcome.
And as for those plants, that can be explained with Capillary action:
Capillary Action (from Chemwiki)
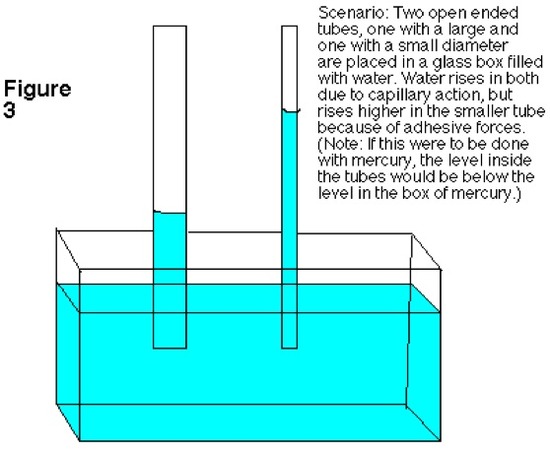
Scientific principles have the knack of explaining abnormal events that occur around us. A liquid, like water, ascending up a narrow tube naturally is not considered as a typical occurrence. Since, water and other liquids in general flows with the gravitational forces, it requires specific circumstances and physical effect to flow against the forces of gravity. This scientific phenomenon is called capillary action. The transportation of water from the roots to leaves of the tree happens due to the underlying principles of cohesion and transpiration.
Introduction
Capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to adhesive and cohesive forces interacting between the liquid and the surface. When intermolecular bonding of a liquid itself is substantially inferior to a substances’ surface it is interacting, capillarity occurs. Also, the diameter of the container as well as the gravitational forces will determine amount of liquid raised. While, water possesses this unique property, a liquid like mercury will not display the same attributes due to the fact that it has higher cohesive force than adhesive force.
Forces in Capillary Action
Three main variables that determine whether a liquid possesses capillary action are:
- Cohesive force: It is the intermolecular bonding of a substance where its mutual attractiveness forces them to maintain a certain shape of the liquid.
- Surface tension: This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in Newton/meter.
- Adhesive force: When forces of attraction between unlike molecules occur, it is called adhesive forces.
Capillary action only occurs when the adhesive forces are stronger than the cohesive forces, which invariably becomes surface tension, in the liquid.
A good way to remember the difference between adhesive and cohesive forces is that with adhesive forces you add another set of molecules, the molecules of the surface, for the liquid to bond with. With cohesive forces, the molecules of the liquid will only cooperate with their own kind. Decreased surface tension also increases capillary action. This is because decreased surface tension means that the intermolecular forces are decreased, thus decreasing cohesive forces. As a result, capillary action will be even greater.

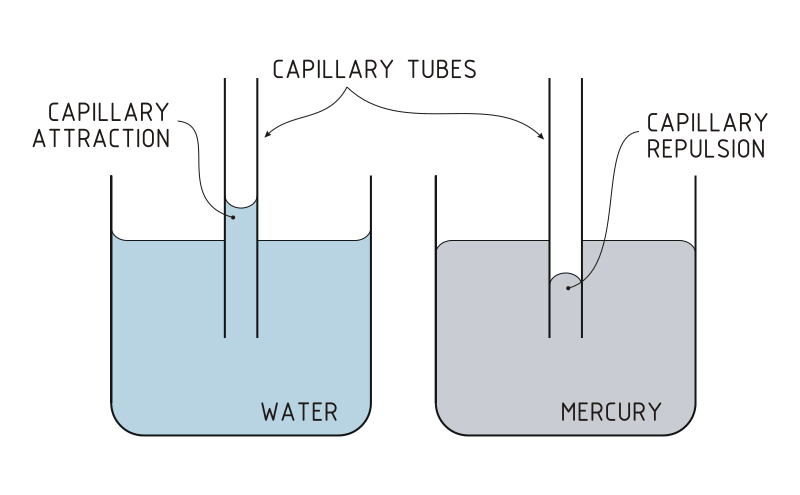
Figure 2:The scientific properties of surface tension and cohesion allow the water strider to calmly walk across water without drowning. http://commons.wikimedia.org/wiki/File:Water_Strider.jpg. Figure 4: It is possible to see that in water, the strength of the adhesive forces are larger than the strength of the cohesive forces. This results in the concave formation of water in the capillary tube; this is known as capillary attraction. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. This is known as capillary Repulsion. http://commons.wikimedia.org/wiki/File:Capillary_Attraction_Repulsion_(PSF)(bjl).svg
So what happens with Surface Tension in Microgravity?
Watch this clip from Plasma Ben:
How this impacts your microgravity experiment design:
And now, as you design your experiments for microgravity, you must consider the differences in bonds. What will happen?
Here are some historical uses of chemical bonding in space exploration:
You may add more historical uses to the Feedback forum at the end of this course.
Here are some other links to articles relating to hydrogen bonding or surface tension in microgravity:
- Effect of microgravity on crystallization
- Condensation of supersaturated hydrogen bonding molecules
- NASA activity on surface tension
- The Dynamic behaviour of surface tension in microgravity
